
The Cottrell Experiment and Diffusion Limitation 3/3
4.9 (207) In stock

4.9 (207) In stock
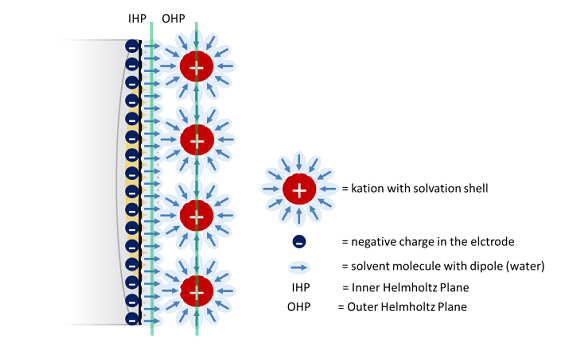
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields

An insight into polyscopoletin electrosynthesis by a quality-by-design approach
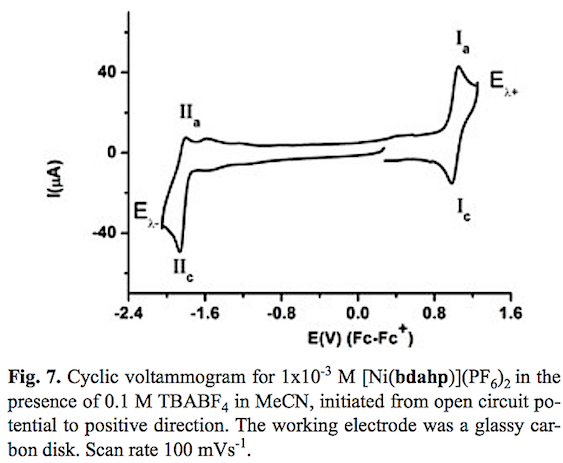
Electrochemical Behavior of Ni(II) Complexes with N2S2 and N6 Ligands as Potential Catalysts in Hydrogen Evolution Reaction

PDF) Time of Flight Electrochemistry: Diffusion Coefficient Measurements Using Interdigitated Array (IDA) Electrodes

The Cottrell Experiment and Diffusion Limitation 3/3

Phase Transformation Lecture 3

ars.els-cdn.com/content/image/3-s2.0-B978012813222

Polymers, Free Full-Text
Crystals, Free Full-Text

support/electrochemical technique

Deep Coupling Network For Multivariate Time Series Forecasting